3x10 for 09/23: AlphaMissense, Immunotherapy, Breast Milk and Breast Cancer
3-sentence summaries of the 10 coolest life science papers out in September 2023
Hi, friends!
Today marks an important day. Life, explained expands with a monthly newsletter called 3x10 (3 times 10) and coming out on the 30th of every month (sorry, February)! In each edition, I will write 3-sentence summaries on the 10 most interesting life science papers that came out that month (subjectively interpretable). 3x10 has a strong bias for innovative approaches in machine learning, cancer research, and genomics.
And now… full speed ahead on a roller-coaster journey through September’s coolest life science papers 🚀🧬!
Enjoy 3x10!
AlphaMissense (Cheng et al., Science): Beyond too much doubt, the star of this month, AlphaMissense from Google’s DeepMind is a deep learning model for predicting the clinical effect (harmful or not) of missense variants (DNA point mutations that change the amino acid sequence of a protein). Combining AlphaFold pre-training with fine-tuning on databases of population frequency of DNA variants in the form of multiple sequence alignments, this model achieves new state-of-the-art performance and is able to reliably classify whether 89% of ALL human missense variants are likely benign or pathogenic. Even though this model is bigger and better than its predecessors (e.g. EVE, ESM), how exactly to employ it in clinical settings remains to be assessed: how reliable and, most importantly, how actionable are AlphaMissense’s variant effect predictions?

1. AlphaMissense, the star of this month. Spatial predictors of immunotherapy response in triple-negative breast cancer (Wang et al., Nature): Despite being humanity’s most promising weapon against cancer, immunotherapy unfortunately only works for some patients, for reasons which remain unclear and non-actionable. This beautiful study analyzes mass cytometry spatial signal of 43 proteins in tumor tissues from 280 triple negative breast cancer patients (the most aggressive breast cancer subtype) enrolled in a clinical trial comparing chemotherapy to chemotherapy plus immunotherapy. It finds that the spatial structure and composition of the tumor and its immune microenvironment both at the start of treatment, as well as early on-treatment, are predictive of patient response to immunotherapy.

2. Spatial predictors of immunotherapy response in triple-negative breast cancer, one of the most beautiful and clear papers I read lately. Early-Stage Breast Cancer Detection in Breast Milk (Saura et al., Cancer Discovery). This short paper is just unbelievable: cell-free tumor DNA shed by breast tumors is detectable via Next Generation Sequencing in breast milk before being detectable in blood plasma, and up to 18 months before diagnosis! You might be rightfully curious how can something like this be rigorously proven: researchers enrolled women with breast cancer diagnosed during either pregnancy or postpartum, and two of the patients had frozen milk samples from either a previous pregnancy (for the patient diagnosed during pregnancy) or as part of surveillance (for the patient with advanced maternal age), which were retrospectively tested (and found positive!) for tumor DNA. Pregnancy and postpartum-related breast cancers are horrific, both psychologically and clinically, and I wholeheartedly hope that such studies will help gather the knowledge necessary to cure this terrible disease once and for all.

3. Early-Stage Breast Cancer Detection in Breast Milk, really a mind-blowing paper. A vertebral skeletal stem cell lineage driving metastasis (Sun et al., Nature). Some cancers (e.g. breast or lung), preferentially metastasize to the spine more than to other bones, for yet unknown reasons (an old theory proposed that coughing pushes malignant cells backward and directs them towards the spine, where they can seed - but nobody proved this). The structure and composition of the spine are unique to vertebrates and result from stem cells different than the ones producing the other bones, named “vertebral skeletal stem cells” (proved in this paper by inserting these cells into the muscles of mice and finding that their progenies resemble spinal structures). By deleting the gene coding for a specific protein secreted by the vertebral stem cells, cancer cells in mice stop spreading to the spine to a large extent (in mice at least), which causally and strongly links this novel stem cell population to the process of tumor metastasis, contributing to our understanding of one of the major killers worldwide.
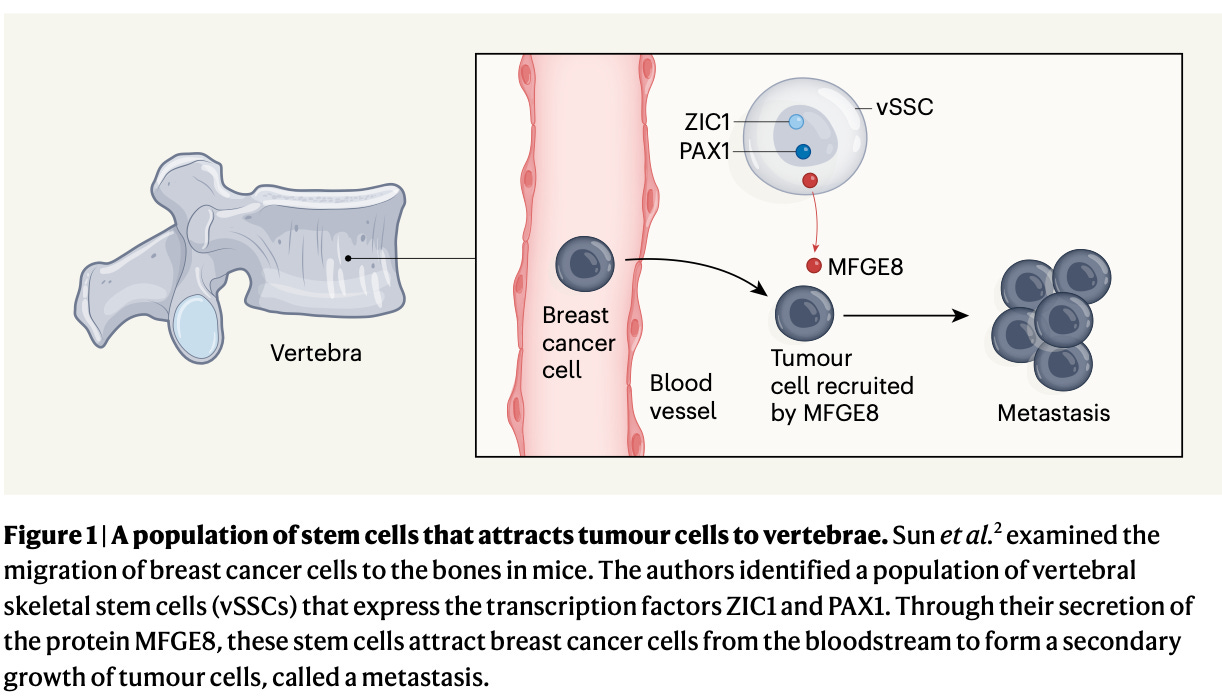
4. A vertebral skeletal stem cell lineage driving metastasis, one of the rare studies which causally and strongly links tumor dissemination to a particular cell population. Mutation rates and fitness consequences of mosaic chromosomal alterations in blood (Watson et al., Nature Genetics). Genomic mosaic copy number alterations are common in tumors and have long been thought to be specific to cancer, but recent research has shown that they exist in healthy tissues as well (sometimes decades before cancer diagnosis), proposing them as a molecular clock to map the continuous temporal development from normal tissues into malignant tumors. Using blood samples from 500,000 people from the UK Biobank, this beautiful population genetics paper disentangles quantitatively the temporal evolution of copy number alterations in the blood of healthy individuals, by quantifying the rates of mutation (i.e., change), selection (i.e., reproductive advantage) and genetic drift (i.e., chance). Through statistical modeling of the relative sizes of the blood clones carrying such alterations in relation to biological age, the authors find that mutations happen relatively rarely, however, their growth advantage can be substantial.

5. Mutation rates and fitness consequences of mosaic chromosomal alterations in blood, a population genetics modeling approach to quantify the mutation rates and fitness advantage of mosaic chromosomal alterations in normal blood. Learning single-cell perturbation responses using neural optimal transport (Bunne et al., Nature Methods). The theoretical framework CellOT aims to understand and predict responses of heterogeneous single cells to chemical, genetic, or mechanical perturbations from unpaired datasets, by learning the maps connecting post-perturbation cells to their pre-perturbation states. The underlying model is an ingenious combination of optimal transport theory and deep learning inference and is applicable to e.g. predict the drug responses of unseen cancer patients from single cell RNA sequencing tumor data. Still, challenges remain for cases in which either patient variability or drug-induced changes are very large - hopefully such challenges will be overcome with more and more data.

6. Learning single-cell perturbation responses using neural optimal transport, an ingenious deep learning modeling framework for drug response prediction from unpaired data. Mismatch repair deficiency is not sufficient to elicit tumor immunogenicity (Westcott et al., Nature Genetics). Back to understanding when and why immunotherapy fails, especially for patients who exhibit higher DNA mutational burden (more mutations), usually associated with improved immunotherapy responses (why? because the immune system finds more enemies - mutated neoantigens - to train and fight against). This paper proposes and analyzes two mouse models of lung and colon cancers with DNA mismatch repair deficiency (leading to a much higher mutational load) and finds - surprisingly! - that the resulting tumors do neither facilitate T cell infiltration nor do they show a strong immunotherapy response. This happens as a consequence of substantial mutational heterogeneity, especially at the subclonal (lower frequency) level, which blunts immunotherapy response and ultimately allows immune evasion.

7. Mismatch repair deficiency is not sufficient to elicit tumor immunogenicity, a landmark study with deep potential clinical implications, elucidating some of the mechanisms underlying immunotherapy response. A foundation model for generalizable disease detection from retinal images (Zhou et al., Nature). RETFound is a ChatGPT-like large language model that extracts incredibly relevant health information from 1.6 million unlabeled images of the human retinas alone. The pre-trained model is then fine-tuned on many downstream clinical tasks with labeled data for detecting various disease conditions (eye disease, Parkinson’s, heart conditions) and shows impressive performance across different ages and ethnicities. Retinal imaging is an even more valuable medical instrument than previously thought, and models built on this data could one day assist and guide medical professionals toward better diagnosis and treatment decisions.

8. A foundation model for generalizable disease detection from retinal images, trained on 1.6 million unlabelled retina images, and fine-tuned on supervised data from various health conditions. A bioelectrical phase transition patterns the first vertebrate heartbeats (Jia et al., Nature). Many people (especially parents!) know that the heart is one of the first organs to function (a baby embryo’s heart starts beating at only 22 days old), but how exactly the absolute first heartbeat arises in a previously silent tissue remains a fascinating mystery. This paper did sophisticated live imaging on zebrafish embryos and managed to capture fascinating videos of hearts blinking for the first time. Large-amplitude Ca++ spikes (responsible for the beats) seem to emerge in all cells at once in a concerted tissue-level and phase-transition-like manner, driven however by a quasi-random origin “leader” cell that first develops spontaneous activity, further “inspiring” neighboring cells to gain synchronized frequencies (how amazing is that).
A wearable aptamer nanobiosensor for non-invasive female hormone monitoring (Ye et al., Nature Biotechnology). Hormones are notorious for fluctuating wildly even throughout the day, and blood tests only offer invasive and static snapshots into hormonal activity, limiting our understanding of hormones’ dynamic impact on health and well-being. This paper introduces a novel wearable device that monitors estradiol (estrogen) non-invasively from sweat: a bendable wrap that sits on the finger, just like a ring, and sends data wirelessly to a smartphone. The measurements of this device are validated in human participans, and backed up by a direct correlation between sweat and serum estradiol levels, as well as clear cyclical fluctuation trends in sweat estradiol levels during the menstrual cycle.

10. A wearable aptamer nanobiosensor for non-invasive female hormone monitoring, a cool new gadget. That was 3x10 for September 2023. It you have suggestions of cool papers to include in October’s 3x10, please reach out either via Substack (Chat or Notes) or Twitter.



Great post - thanks for doing this. Looking forward to more!
thanks! glad you enjoyed it