3x10 for 11/23: The weather, the immune system, deep learning and pregnancy
3-sentence summaries of the 10 coolest life science papers out in November 2023
Hi, friends!
The world is full of stochasticity, and a little bit of it has spilled over the delivery date of our November digest as well (🤒🤧). But the wait is finally over, and November’s roller-coaster 3x10 is now here. In this edition, you’ll read 3-sentence summaries of papers on deep learning advances in both weather prediction and the detection of pancreatic lesions from images, detailed mechanistic description of how cancers hijack neurons and the immune system to their benefit, synthetic genomes, causal inference, new neurons in pregnancy, and more 🚀🧬.
Enjoy 3x10!
Weather prediction finally getting real (Lam et al., Science). One of the longest-standing arguments against the usefulness of computational modeling in general (and deep learning in particular) has now just crumbled: “we can’t even properly predict the weather” - thanks to DeepMind, now we can. The model, GraphCast, is implemented as a neural network architecture (and based on graph neural networks), can be ran on a desktop computer, and produces super fast (<1 minute) and super accurate weather predictions. The model is first trained using estimates of past global weather from 1979 to 2017 made by physical models, then it uses both the current state of the weather, as well as weather estimates of the past 6 hours, in order to predict the weather 6 hours ahead - and this estimation trick is used to successively push predictions further into the future.
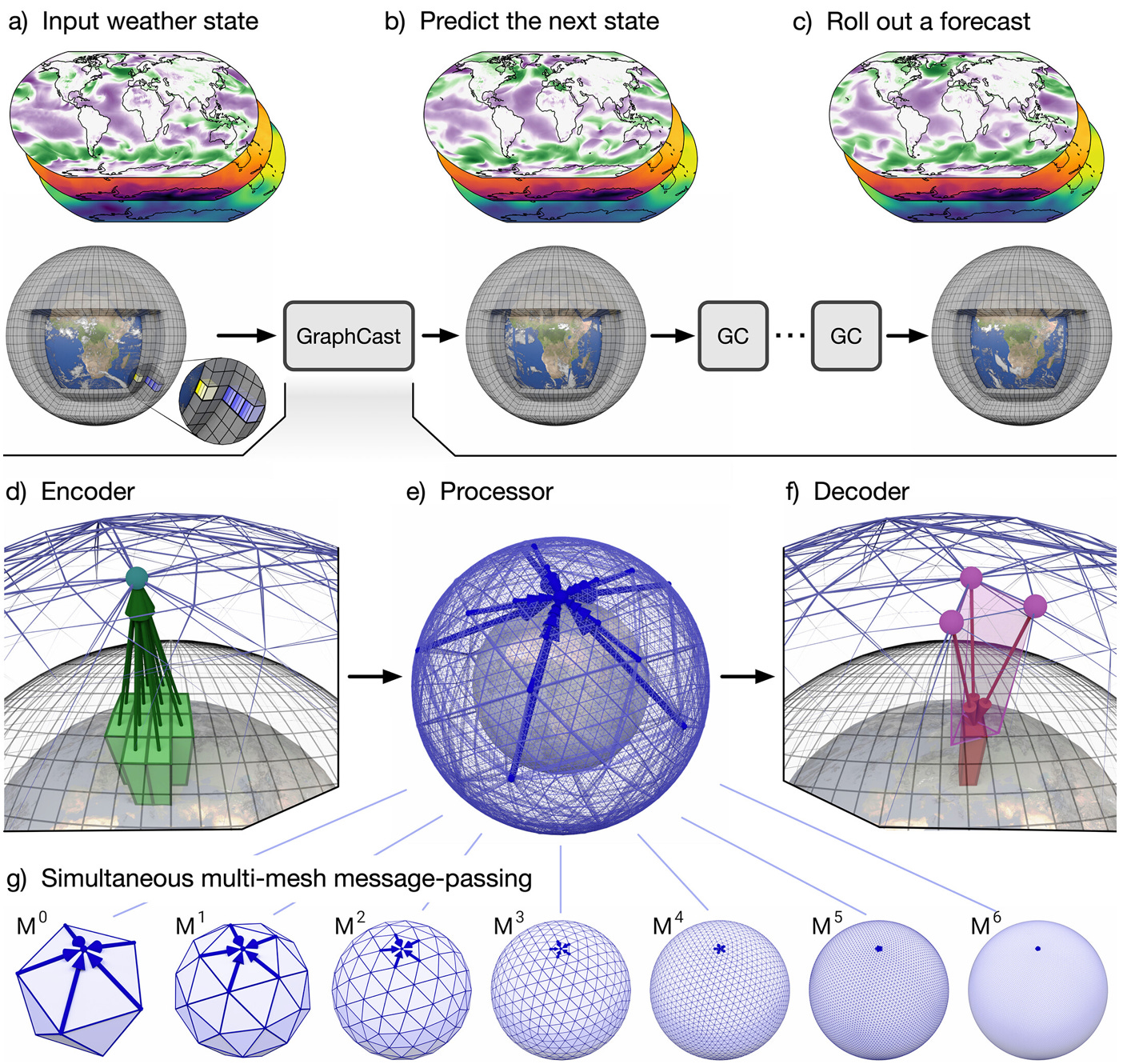
1. GraphCast: fancy (and finally accurate!) weather prediction. Weather forecasting will never be the same again - what a relief. How CAR-T cells can become unfriendly (Lareau et al., Nature). Anti-cancer chimeric antigen receptor (CAR) T cells are first extracted from cancer patients, artificially engineered to fight against cancer cells in a targeted and precise manner, and then infused back into the patients. Unexpectedly, a very rare population of these T cells (1 in 300 - 10,000 cells) can sometimes become “super-expressors” of the human herpesvirus 6, a virus which in some cases causes brain inflammation and can be very dangerous. This study offers a mechanistic justification for anecdotal medical reports, and will hopefully inform the future design and improvement of CAR-T therapies, such that this newly identified potential complication is circumvented.

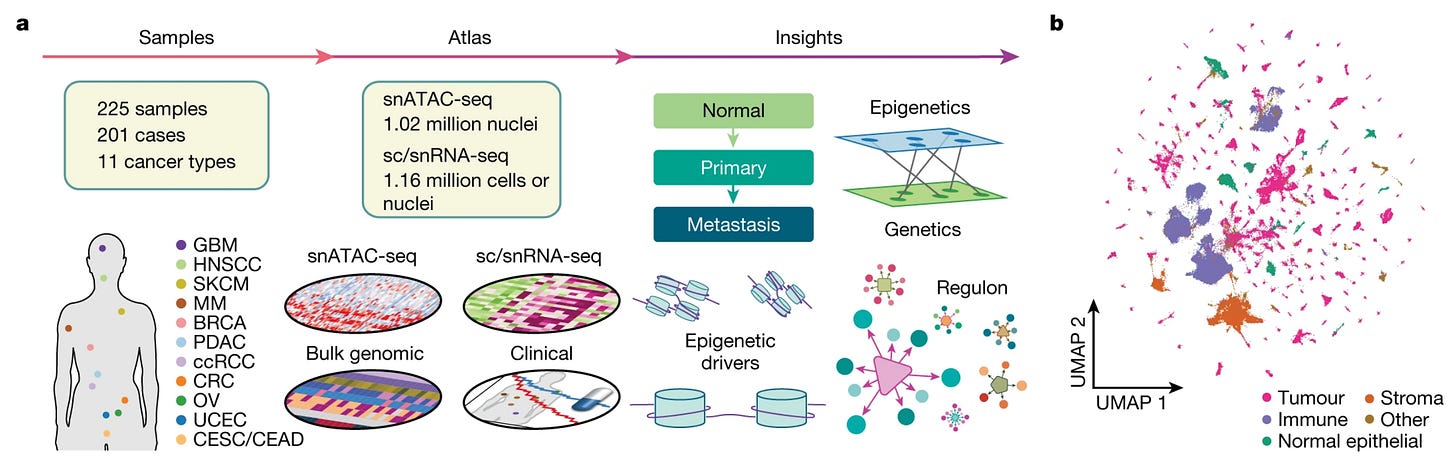
2. Human viruses can get out of latency and become reactivated in conditions of acute stress. This paper finds that the human herpesvirus 6 can sometimes undergo this exact process in artificially engineered T cells used in immunotherapy as cancer treatment (bad news!). Epigenetic regulation during cancer transitions across 11 tumour types (Terekhanova et al., Nature). It has become increasingly clear over the past few decades that not all alterations driving cancers are genetic, and that the epigenome does influence cancer initiation and progression in essential ways. This new study has generated single-cell chromatin accessibility data for 1,000,000 cells from 200 tumor samples of 11 different cancer types, using single-nucleus assay for transposase-accessible chromatin (a large part of which was also paired with scRNAseq expression profiling). Valuable dataset for the community to mine further and, as expected, some regulatory regions called as drivers are unique to a single cancer type, whereas others are shared among types.
Living and replicating yeast genome with over 50% synthetic DNA (Zhao et al., Cell; Schindler et. al, Cell; Taghon et al., Cell Genomics). The genome of Saccharomyces cerevisiae (aka yeast) has 16 chromosomes: in this new artificially-designed strain, 6.5 of them were synthesized in the lab, and an additional chromosome was created by combining various edited bits and pieces. Doing this is not easy, and the Sc2.0 consortium (which consists of labs from all around the world) has been working towards the goal of creating a fully artificially engineered yeast for the past 15 years. A main hurdle to overcome was eliminating potential sources of instability in the yeast genome, by removing repetitive noncoding DNA regions, as well as relocating regions that code for transfer RNAs from their original positions spread across the genome into a new specialized synthetic chromosome (wow, this sounds totally crazy).

4. Design, construction, and functional characterization of a tRNA neochromosome in yeast: a mind-blowing paper describing how researchers constructed a designer chromosome that functions as an additional, de novo counterpart to the native complement of Saccharomyces cerevisiae. Pregnancy transiently remodels olfactory function in anticipation of motherhood needs (Chaker et al., Science). Lots of things happen during pregnancy, which remains one of life’s deepest mysteries, but the birth of new neurons isn’t necessarily something most people would expect. This cool study shows how during mouse pregnancy, multiple neural stem cell pools are activated in mothers and generate specific olfactory bulb interneurons that function around birth to modulate aspects of maternal care, including own-pup recognition, and then disappear as pups mature. This evidence contributes to understanding how the heterogeneity in neural stem cells might provide a substrate for adaptive brain plasticity in response to different physiological states (nice Twitter/X thread).
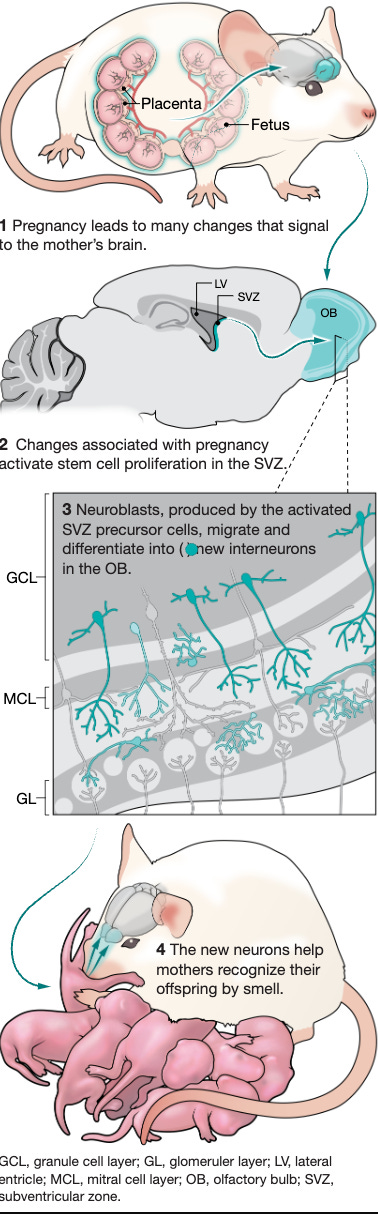
5. Pregnancy transiently remodels olfactory function in anticipation of motherhood needs: in mice, giving birth to pups also means new neurons to recognize these pups. This paper comprehensively describes the mechanisms by which this crazy process happens. Deep learning models outperform radiologists in detecting pancreatic lesions in multiple medical centers (Cao et al., Nature Medicine). Pancreatic cancer is a very aggressive disease with a poor prognosis, usually diagnosed too late and in urgent need for effective early detection methods. This study describes PANDA, an AI imaging-based screening tool, trained on more than 3,200 image sets (70% of which from patients with a pancreatic lesion) and validated in a multi-center cohort of 6,200 patients, as well as a second cohort with 20,000 patients from the same institution. The algorithm achieved a sensitivity of 92.9% and a specificity of 99.9% for the identification of pancreatic lesions, with an area under the curve (AUC) of 0.986–0.996, and identified pancreatic adenocarcinomas better than radiologists.
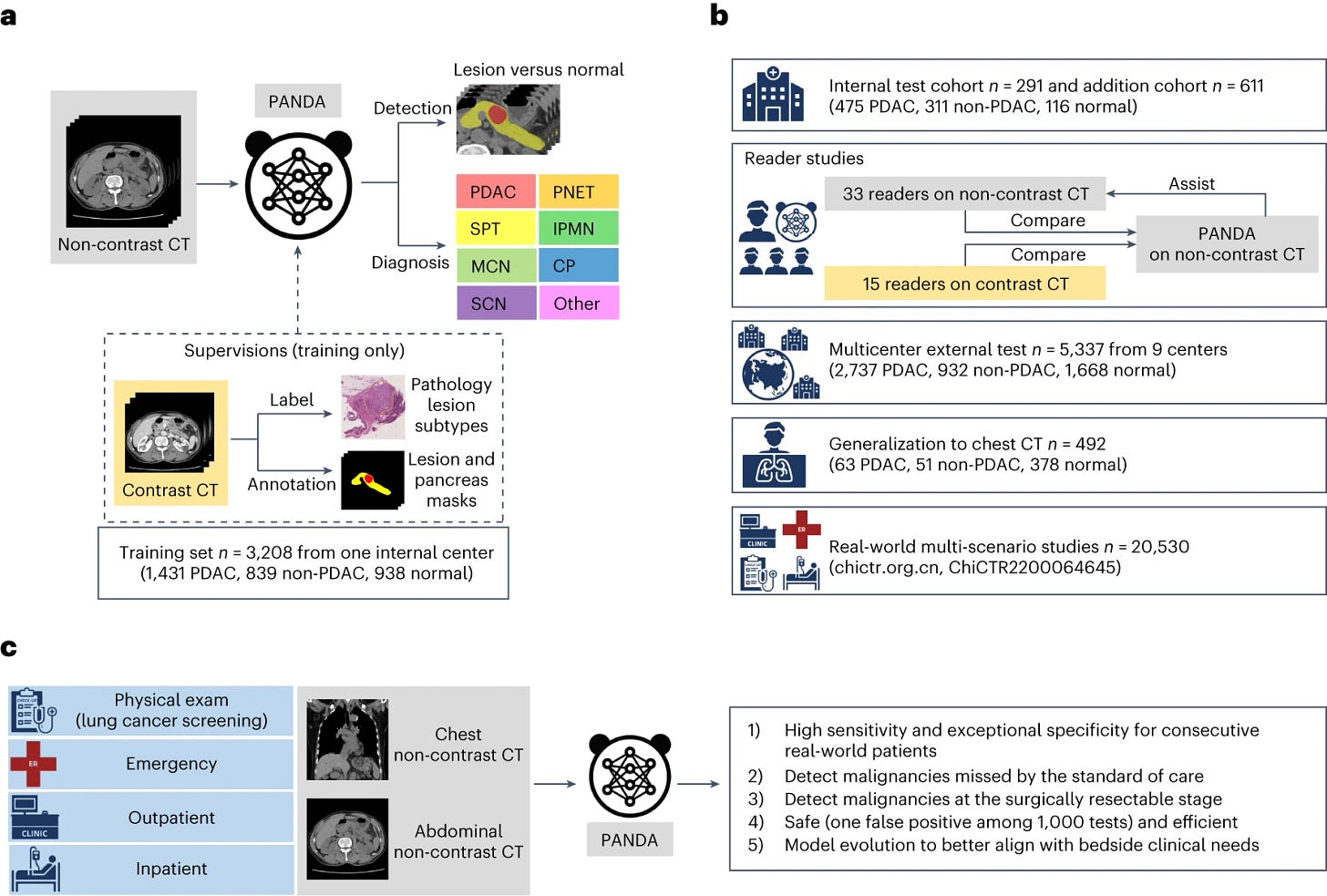
6. Overview of PANDA’s development, evaluation and clinical translation. PANDA takes non-contrast CT as input and outputs the probability and the segmentation mask of possible pancreatic lesions. Brain tumors hijack plasticity mechanisms to enhance cancer growth and ensure survival (Taylor et al., Nature). This important paper carefully characterizes the way in which brain tumors use a previously described neural plasticity mechanism which boosts neuronal activity (TRKB & BDNF related signaling) to malignant purposes. Specifically, BDNF supports the proliferation of malignant brain cells in mice transplanted with human cells and in cancer cells grown in vitro. Knowing that the survival and malignancy of brain tumor cells are boosted by their ability to hijack neural mechanisms that increase synaptic strength is not a good news - but hopefully therapies targeting these neural interactions can be employed to slow down brain cancer growth.

7. Connections between brain cancer cells and neurons as examined in great mechanistic detail in the paper by Taylor et al. This graphic comes from the very comprehensive News & Views article on the topic. Another bad news: macrophages can also support tumor cells, this time in pancreatic cancers (Caronni et al., Nature): a specific population of tumor-associated macrophages (TAMs) which express interleukin-1β (IL-1β) is responsible for sustaining an inflammatory loop together with tumor cells. Following lots of experiments and genomic single cell and spatial analyses, this reprogramming process was found to happen early in tumor development and to be distinguished by lasting transcriptomic changes in tumor cells, as well as specific spatial patterns, further leading (or at least being associated with) poorer patient outcome. On a potential future therapeutic ray of hope, blocking the activity of interleukin-1β helped to control the growth of pancreatic tumor cells (in mice).

8. IL-1β+ macrophages fuel pathogenic inflammation in pancreatic cancer: IL1B+ TAMs correlate with poor prognosis in human pancreatic ductal adenocarcinomas and are conserved in mouse models (Figure 1). Extensive single cell resource on immunaging and how the immune system changes with age (Terekhova et al., Immunity): profiling 2 million single cells with single cell RNA sequencing and TCR/BCR sequencing of 317 samples from 166 healthy individuals aged 25–85 years old. 12 out of the 55 identified subpopulations change with age: type 2 memory CD4+ and CD8+ T cells increase with age and can produce more IL-4, NKG2C+GZMB–XCL1+ defines a unique memory CD8+ T cell subset that decreases with age and HLA-DR+ CD4+ memory T cells and GZMK+ CD8+ T cells accumulate with age. A healthy and potent immune system is absolutely essential for healthy aging, so understanding the natural changes that immune cells undergo with age is incredibly important for increasing healthspan and lifespan.

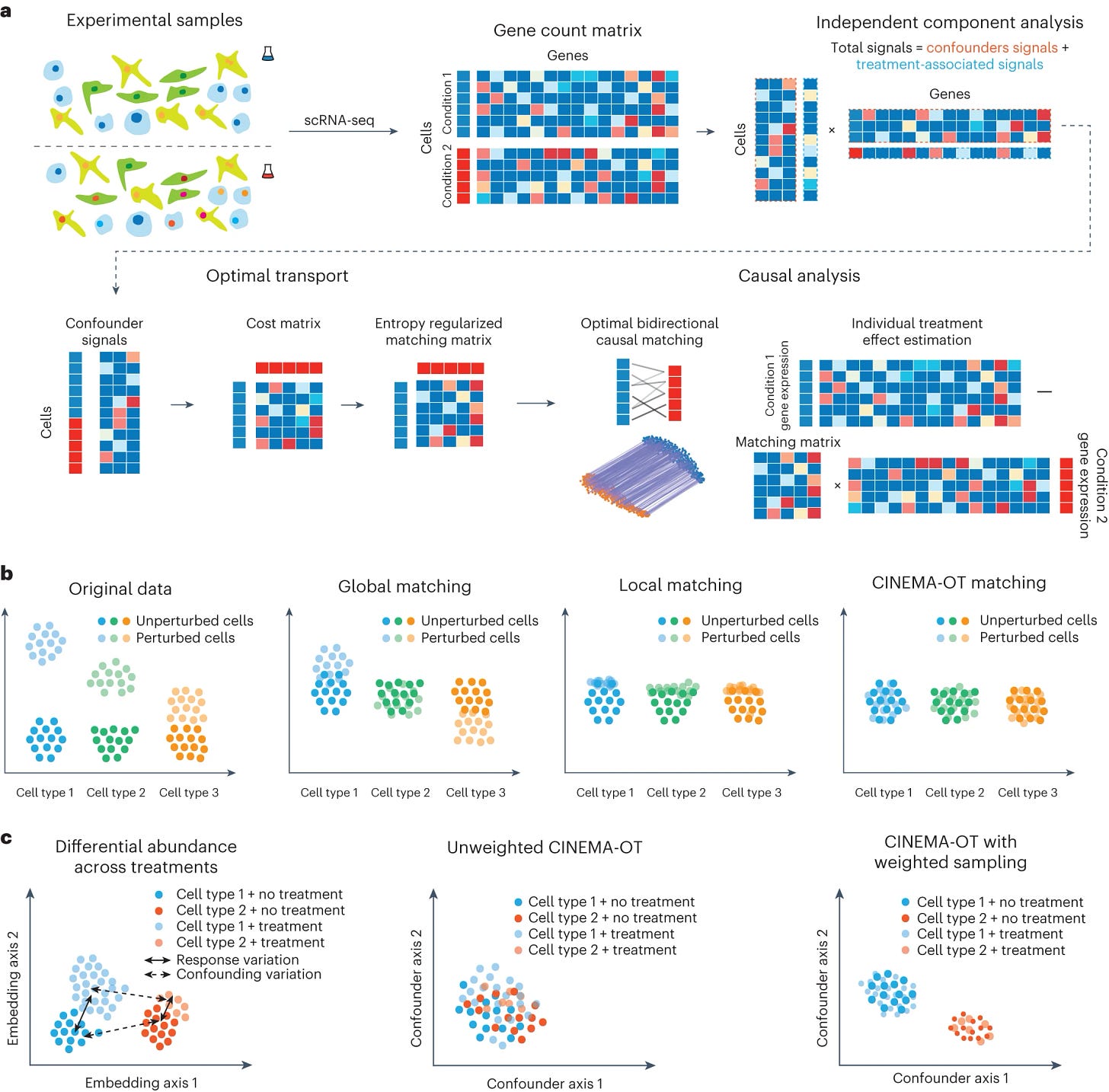
9. Immune aging atlas of 2 million cells: great resource! Understanding how and why the immune system loses its potency during aging is essential for staying health for longer. Finally, CINEMA-OT, a very nice new computational framework based on causal inference for single cell perturbation analysis (Dong et al, Nature Medicine) , has been used to investigate the cytokine language that activates immune response, revealing how different immune cells use combinations of cytokines to communicate to each other. Certain cytokines combine synergistically, and this combination of signals with novel outcomes acts as a language which directs the actions of the immune cells. The algorithmic innovation comes from computationally matching the states of different cells between control and perturbed populations, in order to infer the dynamic changes between the two conditions.



Thanks Simona - great read. Looking forward to the January edition.2
Thanks Simona - great read. Looking forward to the January edition.